Participant information sheet


Introduction
Thank you for taking the time to read this information.
We are inviting you to take part in a study to help us understand if, how, and why, ethnicity affects the immune response to COVID-19 infection and vaccination in those working in healthcare settings. This research is part of a larger project called “UK-REACH: United Kingdom Research Study into Ethnicity and COVID-19 outcomes in Healthcare workers”. This study will also investigate the effectiveness of the seasonal influenza vaccination and will build on work from the BELIEVE study which ran during the 2020/21 flu season.
Before you decide whether to take part, it is important that you understand why the research is being done and what it will involve. Please read the following information carefully and discuss it with others if you wish. If you have questions, please contact us at direct@leicester.ac.uk or on 07425 611865.
What is the purpose of the study?
Previous research has found that healthcare workers and those from ethnic minority groups are at high risk of COVID-19 infection and may be at higher risk of severe disease than their white colleagues. We know that immune responses are key to the outcome of COVID-19 infection and vaccination.
BE-DIRECT aims to determine whether there are differences in immune responses to COVID-19 infection and vaccination (including booster vaccination) between ethnic groups in order to understand whether these differences may contribute to the observed differences in infection risk and COVID-19 outcome in different ethnic groups.
It is important that the seasonal influenza vaccine offers effective protection against infection throughout the influenza season which is usually between December to May in Leicester. Data is limited but evidence suggests that the effectiveness of the vaccine may be lower in people that have received previous influenza vaccinations. We will also investigate the effects of giving the flu jab together with the COVID-19 booster.
We will achieve this aim by combining information you provide us with on a questionnaire with information from your health record and by collecting blood samples. The blood samples will be analysed:
- to look at how your white blood cells(cells that fight infections) respond to COVID-19 infection or the COVID-19 vaccine.
- to assess the levels of proteins in your blood that may be related to immunity to COVID-19
- to find out if you have antibodies against COVID-19 and whether the levels of these decrease over time.
- to examine your DNA to see if we might identify any genetic associations with COVID-19
- to assess the levels of influenza antibodies and whether the levels of these decrease over time
- to look at how your white blood cells (cells that fight infections) respond to influenza infection or the influenza vaccine.
Why have I been invited to take part?
We are invited you to take part in this study as you:
- Are aged 16 or over
- Live and work in the UK
- Are a healthcare worker (including student healthcare workers) or work in a healthcare setting in Leicester or the surrounding area (Note: You do not need to look after patients directly).
- Have previously taken part in the BELIEVE or DIRECT studies
We welcome people from all communities to join the study. Minority ethnic and migrant communities have had higher rates of COVID-19 hospital admissions, and we encourage people from these communities to consider taking part.
You do not need to have had COVID-19 infection or vaccination to join the study– it is important for us to include people who have and have not been infected or vaccinated.
You do not need to look after patients directly to join the study.
You can still take part even if you have already had your COVID-19 booster
What will happen if I decide to take part?
We will ask you to:
- Provide consent to participate in the study if you are happy to do so.
- Register your details (including contact details and date of birth) and provide your consent online on our secure web page.
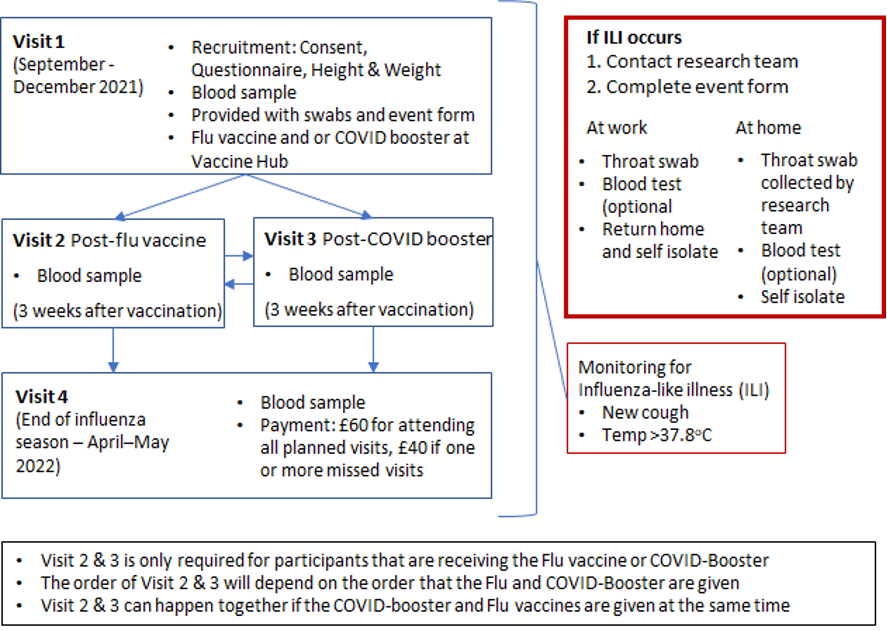
- You will be asked to complete 2 questionnaires during the course of this study, the first of these will take around 10 - 15 minutes. If you are having a booster vaccine, the questionnaire will be completed in your pre-booster appointment. If not having a booster vaccine this information will be collected at the next blood test visit (approximately 6 – 9 months after your last COVID-19 vaccine dose, or 6 – 9 months after enrolment if unvaccinated). The second will be completed at your final study visit. We will ask some basic information about you, your ethnicity, plus whether or not you think you may have had COVID-19 or been vaccinated against it, other aspects of your physical health, occupation and working life, home environment and living circumstances. We will also collect a height and weight measurement.
- A trained member of the research team will then take your height and weight and collect some blood samples that we will use to examine elements of your immune system. In total, approximately 50ml of blood will be taken (around 3 tablespoons worth). The majority of the blood samples will be collected at Leicester Royal Infirmary, however, for staff unable to travel to this site we can offer limited appointments at Leicester General Hospital or the Glenfield Hospital depending on preference.
- You will also be given a pack to be used if you develop an influenza like illness (ILI). This pack will contain a thermometer & an event form to answer about the symptoms you are experiencing.
- If you develop an influenza like illness (temperature above 37.8 oC and new cough) during the study we will ask you to complete the event form & contact the research team. The research team will then arrange for a throat swab to be taken whilst you are isolating. Collection will be by a healthcare worker trained in the use of personal protective equipment for COVID-19 and you will be called with the results of the throat swab.
- We will contact individuals who have registered for the study, by telephone, text message or email to prompt them to complete questionnaires and/or arrange for their blood sample to be taken.
- Study visits and ILI monitoring are summarised in the figure below
-
If you plan to have an Influenza or COVID-19 booster vaccination we will collect blood samples at the
following timepoints:
- Up to 14 days before vaccinations
- 3 weeks after COVID-19 booster vaccination (+/-1 week)
- 3 weeks after seasonal Influenza vaccination (+/- 1 week)
- April - May 2022
- Nb. Visits 2 and 3 can happen at the same time if COVID-19 booster and the influenza vaccine are given at the same time
- If you are a current participant in the DIRECT study and have had a blood test (either baseline or follow up) in the 2 weeks prior to your COVID-19 booster then this will be used as your pre-booster sample for the BE-DIRECT study. If this is the case then your BE-DIRECT consent, questionnaire and height and weight measurement will be undertaken at the post-booster appointment.
- If you have already had a COVID-19 booster but have not had a pre-booster blood sample collected then you are still eligible for the BE-DIRECT study and your first visit will be 3 weeks after your COVID-19 booster in which we will undertake consent, a questionnaire, a height and weight measurement and a blood sample.
-
If you do not want an Influenza or COVID-19 booster vaccination we will collect two blood samples at the
following timepoints:
- September – December 2021
- April - May 2022
- We will collect blood at follow-up visits around 10 - 50ml depending on the visit.
- We ask that you inform the research team if you develop an influenza like illness (temperature above 37.8 oC and new cough) during the course of the study. Influenza like illness share the same symptoms as COVID-19. It is therefore important if you develop an influenza like illness that you and your household follow the latest government advice about self-isolation which can be found online. https://www.gov.uk/government/publications/covid-19-stay-at-home-guidance/stay-at-home-guidance-for-households-with-possible-coronavirus-covid-19-infection
- We would like for you to contact us or for you to give us permission to contact you to arrange collection of a further blood sample if you develop confirmed or suspected COVID-19 within the timeframe of this study. Researchers will contact you to arrange collection of the blood sample whilst you are isolating. Collection will be by a healthcare worker trained in the use of personal protective equipment for COVID-19. However, this is optional and you can indicate on your consent form if you do not want to take part in this aspect of the study.
- We will periodically link to your electronic health records for up to 25 years. This will be done in the NHS and your information will remain anonymous to the research team.
- We will also link to COVID-19 related outcomes in your occupational health record (this will include COVID-19 related absences from work, COVID-19 swab results and COVID-19 antibody results). Your occupational health record will not be accessed by researchers, instead this information will be provided by the occupational health department and only COVID-19 and influenza related information contained within this record will be provided.
Do I have to take part?
You do not have to take part – it is up to you to decide whether or not you would like to take part. If you do decide to take part you are still free to withdraw at any time without giving a reason.
If you decide to take part, you will be asked to complete a consent form once you have had the opportunity to read this leaflet and ask any questions you might have. You will be able to download an electronic copy of your consent form to keep for your own information.
If you were originally part of the BELIEVE study and choose not to take part in BE-DIRECT your participation in BELIEVE will be complete as there are no further BELIEVE appointments.
If you were originally part of the DIRECT study and choose not to take part in BE-DIRECT you will have an appointment as planned for the DIRECT study in 6-9 months after your most recent COVID-19 vaccination or 6-9 months after enrolment if you have not received the COVID-19 vaccination.
What are the possible benefits of taking part?
- This research could help to improve understanding of the causes and consequences of COVID-19 infection and responses to COVID-19 vaccination.
- If a new treatment or test were developed, there would not be a financial benefit to you.
- In recognition of your time and any expenses incurred all participants will receive £60 for attending all planned follow up appointments and £40 if they miss one or more appointments. This will be payable at the final visit.
What are the possible disadvantages of taking part?
Completing the initial questionnaire will take about 10 - 15 minutes. The collection of blood samples may cause mild discomfort and could leave a small bruise.
Who will be able to use my data?
We may make available anonymous information from the study, labelled only with unique codes to researchers approved by the UK-REACH Study Steering Committee in discussion with the Chief Investigator and UK-REACH Core Management Group. This information will not identify you.
How will my data be used?
Although information including name and contact details will be kept on record, these data will only be used for two specific purposes:
- Firstly to allow secure linkage of the questionnaire data to your healthcare records and COVID-19 related outcomes in your occupational health record
- Secondly for future contact by the BE-DIRECT team. A very small number of study team members will have access to your name and contact details, so that we can re-contact you to arrange a follow up appointment, for further questionnaires or for other aspects of the study.
People who do not need to know who you are will not be able to see the data collected during the registration process (including name and contact details). Your data will have a code number instead and stored separately from the questionnaire data.
We will ask for your permission to periodically link your questionnaire responses to data from your medical records (including data on COVID-19), from COVID-19 research study symptom trackers and apps (if you use these) and from other research studies you may be participating in. Linkage to your health records is important as it will allow us to understand the long-term effects of the pandemic on your health as well as any pre-existing conditions that might impact upon your immune system.
Linkage to your healthcare record is highly secure and can be summarised as follows:
- The personal information you provide to BE-DIRECT is used by NHS organisations to identify your NHS number
- Your NHS number is used by NHS organisations to link to your healthcare records
- Once your records have been located all identifiable information is removed (name, date of birth, address, NHS number)
- De-identified healthcare records attached to unique codes are then transferred securely back to UHL
- Your questionnaire data – which has no personally identifiable information and is linked to your unique code is stored at the University of Leicester and securely transferred to UHL.
- Your blood samples will also be labelled with your unique code and, when analysis of the samples is complete, the laboratory data will be transferred securely to UHL and linked with your questionnaire data and healthcare records.
- Any link between the unique codes and your personal details will be stored separately at the University of Leicester.
Linkage to COVID-19 related outcomes (absences, swab test results, antibody results) in your occupational health record will be performed by a member of the occupational health department at UHL (not by researchers), and is strictly limited to information about COVID-19 and Influenza.
This process means that when researchers are analysing the data to answer research questions, they will not be able to identify you and that healthcare records with your personal details attached never leave the NHS.
We will not share any information about your health, or any other information you have given us, with your employer or professional body.
Once we have finished the study, we will keep some of the data so we can check the results. We will use the information you provide in reports and publications, for example, in scientific journals. This will be anonymised, meaning that we will write our reports in a way that no-one can work out that you took part in the study.
Access to any identifiable data (e.g. name, address) will be limited to select members of the research team and authorised individuals from the NHS or from the Sponsor (University of Leicester) or regulatory authorities. We expect to store the data for a period of 25 years, although this period will be reviewed by an expert Scientific Committee.
Information from the study, labelled with only a unique identifier (and therefore not identifiable as coming from you), will be made available to other approved researchers. This could include researchers in other countries and in commercial companies.
What are my choices about how my information is used?
You can stop being part of the study at any time, without giving a reason. If you wish to withdraw from the study we would like you to optionally complete a withdrawal form. This is so we are clear whether or not we can continue to access your electronic healthcare records. If you do not wish to complete a withdrawal form, you can contact the study team by telephone or email (using the details provided below). We would like to continue to access your health records for up to 25 years, however, if you do not want this to happen then you can tell us on the withdrawal form or by contacting the study team by email or telephone (using the details provided below). If you do decide to withdraw then any information already collected will remain and be used in the study. No further data collection will be performed and we will not contact you again about this study.
We need to manage your records in specific ways for the research to be reliable. This means that we won’t be able to let you see or change the data we hold about you.
Where can I find out more about how my information is used?
You can find out more about how we use your information:
- at www.hra.nhs.uk/information-about-patients
- by asking one of the research team
- by sending an email to uk-reach@leicester.ac.uk
- by ringing us on 07425 611865, or
- by contacting the University of Leicester Data Protection Officer via email to ias@le.ac.uk.
Storage and future use of samples
- At the end of the study (which is in 25 years), with your permission, we would like to keep any remaining samples (in their pseudonymised form) for use in future ethically approved research.
- For this type of study, we will store the anonymous research data and any research documents with personal information, such as consent forms, securely at the University of Leicester for 6 years after the study ends If you give us permission to retain your samples for future research, it is necessary to retain your consent form until the samples have been depleted or destroyed, or if you withdraw your permission. This may be longer than 6 years.
- The Human Tissue Authority is the regulatory authority responsible for the oversight and inspections of human tissue storage in the UK after a study has concluded. We require your consent form to comply with the Human Tissue Authority to ensure we have obtained your permission to retain the samples beyond the life of this project. Your consent form would be stored independently from your pseudonoymised samples to ensure your samples remain anonymous to researchers that may use them in the future.
- If your samples are used up within this period your consent form and personal information will be destroyed 6 years after the study ends.
Will I receive results of tests on my DNA?
Information relevant to you or your family might be discovered by the study of DNA. These measures are useful for research, but they are not the same as clinical genetic tests. Research genetic measures are not of a high enough standard for diagnosis. Therefore, we will not routinely provide you with the results of all the genetic variants measured in the study. In rare situations we may advise a participant to seek medical advice based on a research result for a specific genetic variant for which there may be medical treatment. For example, one which causes very high blood cholesterol which might be lowered by medication. A Scientific Committee based on expert advice will decide what is considered to be a genetic variant that can be medically treated. On the consent form, we will ask you to choose whether you would wish to be contacted if we do find such a variation. The Clinical Genetics Department at the Leicester Royal Infirmary have a dedicated genetic counselling clinic for patients where genetic variations have been identified in a research study. This will offer advice to patients on the significance of what has been discovered and the steps the patient can take to reduce the likelihood of this having an impact on health in the future.
However, it is important that you understand that we cannot identify all genetic variants for which there may be a medical treatment and our ability to continue the study and to contact participants will be subject to further funding. As a research study we do not have the resources that a screening programme has, and the research genetic measures are not of a comparable standard to genetic testing that is a part of a screening programme. It is important you understand that this study is not a substitute for medical screening, clinical advice or clinical care.
What if something goes wrong?
It is unlikely that you will be harmed by taking part in this study. If you wish to complain about any aspect of the way in which you have been approached or treated during the course of this study, please contact UK-REACH project manager Claire Garwood (cjg29@le.ac.uk; 07425 611865) who will acknowledge receipt of the complaint, investigate, and report back to you within a reasonable period of time.
If something does go wrong and you are harmed during the research and this is due to someone’s negligence then you may have grounds for legal action for compensation against the University of Leicester but you may have to pay your legal costs. The normal NHS complaints service will still be available to you (if appropriate).
Will the findings of the research be published?
We will publish findings from the research in scientific journals. We will summarise published research on a study website: www.uk-reach.org.
Information that you provide in the questionnaires, including quotes, may be used in publications although they will be anonymous.
Who is organising and funding the research?
This research is led by Dr Manish Pareek at the University of Leicester. The study has been supported by funding from UK Research and Innovation.
How was the study reviewed?
This study has been reviewed and approved by an independent group of people called a Research Ethics Committee (Brighton and Sussex) and by the University of Leicester as Sponsor. All research that involves NHS patients or staff, information from NHS medical records or uses NHS premises or facilities must be approved by an NHS Research Ethics Committee before it goes ahead. Approval does not guarantee that you will not come to any harm if you take part. However, approval means that the committee is satisfied that your rights will be respected, that any risks have been reduced to a minimum and balanced against possible benefits and that you have been given sufficient information on which to make an informed decision
Thank you for reading this information sheet.
For further information, please contact the DIRECT / UK-REACH study team:
Email: uk-reach@leicester.ac.uk
Tel: 07425 611865